Abstract
Allogeneic stem cell transplantation (HSCT) is a curative treatment for adult patients with hematologic malignancies, but is limited by severe, life-threatening complications such as acute graft-versus-host disease (aGvHD). We have developed a proteomic urine pattern "aGvHD_MS17", consisting of 17 differentially excreted peptides, capable to predict aGvHD grade II or more (1, 2). In 2008, a multicenter, randomized, placebo-controlled, double blind clinical trial (Pre-GvHD) was initiated testing aGvHD_MS17 for prediction of aGvHD and to initiate pre-emptive therapy using prednisolone 2-2.5mg/kg.
Patients and Methods:
Eleven German transplant-centres contributed 267 patients. Urine was collected weekly from day +7 to +35 and on days +50 and +80 (all +/-3 days) frozen, shipped to Hannover and analyzed using capillary electrophoresis coupled on-line to mass spectrometry (CE-MS) within 72h as described (1). aGvHD_MS17 was considered positive, when the dimensionless classification factor (CF) was +0.1 or more. Eight patients were excluded from analyses, either due to no medication (n=5) or protocol violations (n=3) and 92 were randomized according to the positivity of aGvHD-MS17 to receive either prednisolone (2-2.5mg/kg, n=44) or placebo (n=48) for 5 days followed by a taper for 19 days, if no aGvHD occurred. The remaining 167 patients formed the observation group according to pattern negativity.
About half of the patients had acute leukemia (placebo group: n=24/48 (50%), prednisolone group: n=21/44 (50%); observation group: n= 91/167 (54%)) and were in complete remission/chronic phase (CR/CP) (placebo n=23/48 (47%), prednisolone n=27/44 (61%) and observation n=68/167 (41%). The majority was transplanted from matched donors (placebo: n=42/48 (87%); prednisolone: n=37/44 (84%); observation: 146/167 (87%), using reduced intensity conditioning regimens (RIC; 64%), and a calcineurin-inhibitor based GvHD-prophylaxis with MTX or MMF).
Results:
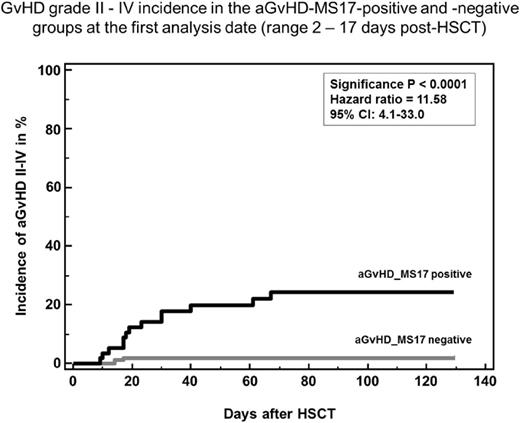
Prospective and blinded evaluation of aGvHD_MS17 revealed that the first analysis time point (day +7; range: 2-17) most accurately predicted aGvHD grade II or more with a sensitivity of 87% and a specificity of 81% prior to clinical signs with a CF of +0.1 (2). Patients with samples positive for aGvHD_MS17 in the early analyses time points had a 21-fold higher risk to develop aGvHD grade II or more ((p<0.0001), Figure 1). By day +28 the predictive value of aGvHD_MS17 was lost. Confounding factors were conditioning with RIC-protocols and early death after HSCT. Patients with one sample positive for aGvHD_MS17 had a 3-fold higher risk to die, 35% of those died prior to day +500, compared to only 10% of the patients with negative samples. Analysis of pre-emptive therapy using prednisolone revealed that the incidence and severity of acute GvHD was not significantly different between the placebo and prednisolone arm suggesting that prednisolone 2-2.5mg/kg was insufficient to prevent aGvHD in this setting. Further analyses of aGvHD according to organ manifestation are ongoing. The occurrence of aGvHD after a positive proteomic pattern test in the placebo group of this trial was lower than in the previous pilot study. Possible reasons include different patient populations ( pilot study: 18% of patients transplanted in relapse; current trial: only 4% transplanted in relapse, increasing the risk to develop aGvHD), different intestinal decontamination and GvHD prophylaxis protocols. The frequency of adverse and serious adverse events was not higher in the prednisolone arm than the placebo arm. No specific safety risk of the pre-emptive therapy with prednisolone was identified.
Conclusions:
Taken together our results indicate that pre-emptive treatment of imminent aGvHD based on proteomic-pattern-diagnostic with prednisolone 2-2.5mg/kg appears to be safe, but did not influence severity or incidence of aGvHD grade II or more. The prospective evaluation of aGvHD_MS17 confirms the highly reproducible results in the early analysis time points (day +7 to +21) for prediction of aGvHD (day +7; range 2-17). Patients with aGvHD_MS17 positive samples have a 21-fold risk to develop severe GvHD (grade II-IV). Moreover, patients with one sample positive for aGvHD_MS17have a 3-fold increased risk of death by day +500 after HSCT.
(1) Blood 2007 :109(12):5511-9.
(2) Leukemia, 2014: , 28(4):842-52
Jochen: Mosaiques GmbH: Employment. Schmid: Jazz: Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding, Speakers Bureau; Novartis: Research Funding, Speakers Bureau; MoilMed: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding, Speakers Bureau. Bethge: Miltenyi Biotech GmbH: Consultancy, Honoraria, Research Funding; Neovii GmbH: Honoraria, Research Funding. Raad: Mosaiques GmbH: Employment. Durban: Mosaiques GmbH: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal